The silver electrorefining process primarily consists of the following key steps:
1. Electrolyte Preparation and Anode Casting:
Electrolyte preparation: Typically, a mixture of silver nitrate (AgNO3), nitric acid (HNO3), and potassium nitrate (KNO3) is used as the electrolyte to enhance its conductivity and adjust the pH value.
Anode casting: Raw materials with high silver content, such as silver alloys or crude silver, are melted and cast into anode plates, which are then hung at designated positions within the electrolytic cell.
2. Electrolysis Setup:
Electrolytic cell and electrodes: An appropriate electrolytic cell is selected, and anodes and cathodes are placed inside. The anode is typically the silver plate that needs refining, while the cathode is often made from highly conductive and corrosion-resistant materials like silver sheets, stainless steel sheets, or titanium sheets.
Power connection: The anode and cathode are connected to the positive and negative terminals of a direct current power supply, respectively, ensuring correct current flow.
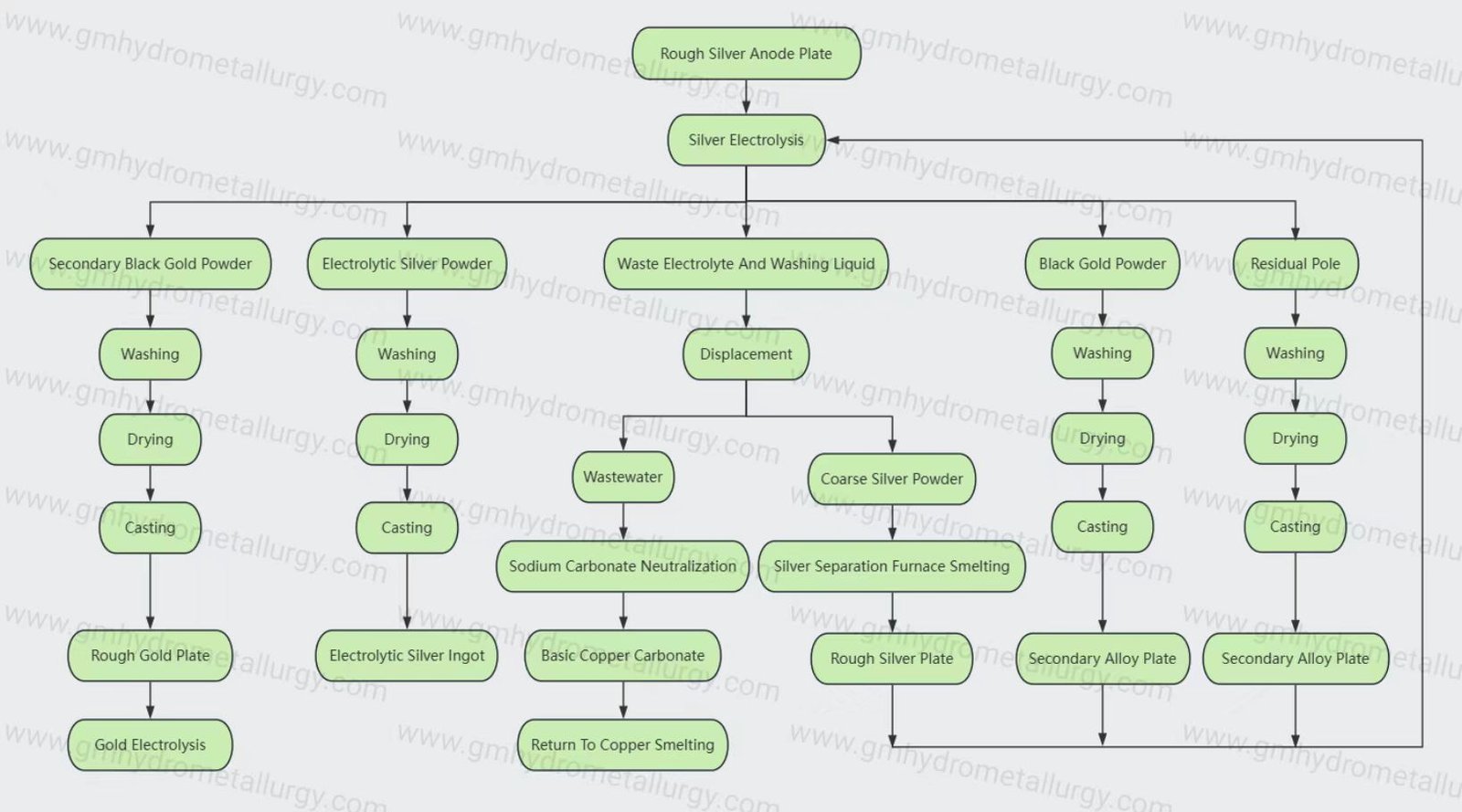
3. Electrolysis Process:
Electrolytic reaction: During electrolysis, elemental silver at the anode loses electrons to become silver ions entering the electrolyte, while other metal impurities may also oxidize and enter the solution; at the cathode, mainly silver ions gain electrons to form elemental silver, which deposits on the cathode.
Control of electrolysis conditions: Parameters such as current density, temperature, and electrolyte circulation must be controlled to ensure efficient and stable electrolysis. Additionally, a stirring rod may be moved back and forth between the anode and cathode to prevent short circuits and promote the deposition of refined silver.
4. Post-Electrolysis Product Handling:
Collection of silver powder: After electrolysis, the deposited silver powder or silver sheets are removed from the cathode, having undergone preliminary refinement.
Further purification: Depending on requirements, the electrolyzed product may undergo further purification and refining to remove residual impurities and achieve higher purity silver products. This can include washing, drying, smelting, and other steps.
In summary, the silver electrorefining process is one where silver is separated from silver-containing raw materials and purified through electrolysis. Precise control of electrolysis conditions is necessary to ensure the efficiency of the process and the purity of the silver product.





